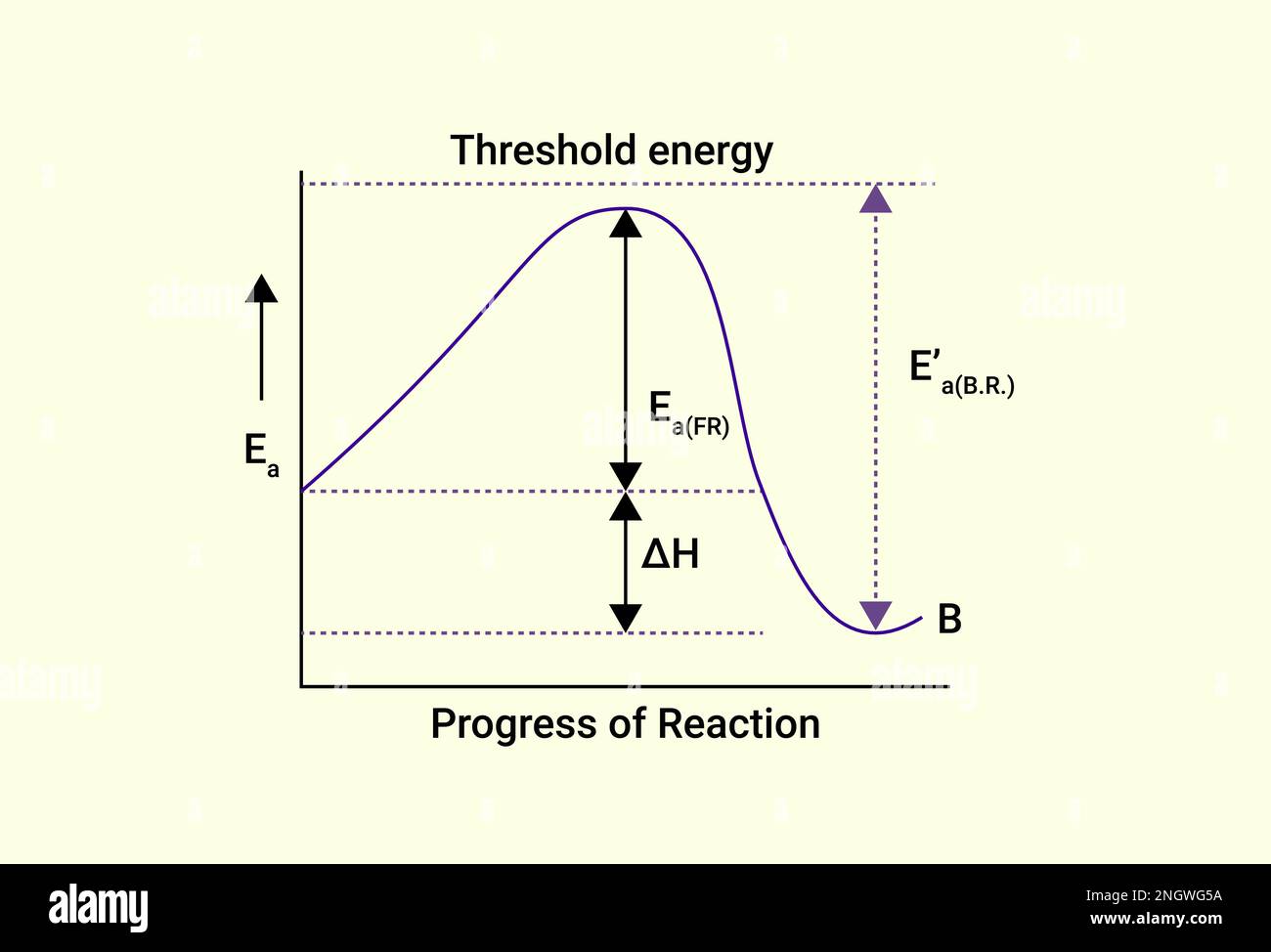
Threshold Energy . threshold energy, or activation energy, is the minimum energy required for a chemical reaction to occur. This equation is valid if the energies are much less than rest mass energies of the involved particles.
from www.alamy.com
This equation is valid if the energies are much less than rest mass energies of the involved particles.in chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they. Threshold energy is the energy of normal.
Graph of Progress of Reaction and Threshold energy Stock Vector Image
Threshold Energy learn the difference between threshold energy and activation energy in chemical reactions. This equation is valid if the energies are much less than rest mass energies of the involved particles.learn the difference between threshold energy and activation energy in chemical reactions. learn the difference between threshold energy and activation energy in chemical reactions.
From www.researchgate.net
The Ikeda diagram [7]. The threshold energies for each configuration Threshold Energy This equation is valid if the energies are much less than rest mass energies of the involved particles.learn how to calculate the threshold energy of a particle reaction using relativistic invariants and kinematics. Threshold energy is the energy of normal. threshold energy, or activation energy, is the minimum energy required for a chemical reaction to occur. Web. Threshold Energy.
From www.researchgate.net
The excitation threshold energy and the slope efficiency as a Threshold Energyin chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they. learn the difference between threshold energy and activation energy in chemical reactions. Threshold energy is the energy of normal.learn how to calculate the threshold energy of a particle reaction using relativistic invariants and kinematics. This equation. Threshold Energy.
From www.researchgate.net
The threshold energy, √ s th , for K + Λ and K + Σ Download Threshold Energylearn the difference between threshold energy and activation energy in chemical reactions. This equation is valid if the energies are much less than rest mass energies of the involved particles. Threshold energy is the energy of normal.learn the definitions and differences of activation and threshold energies, and how they relate to chemical reactions and. learn the. Threshold Energy.
From www.researchgate.net
The displacement threshold energy E pc d in a perfect Cu crystal at Threshold Energylearn the definitions and differences of activation and threshold energies, and how they relate to chemical reactions and. threshold energy, or activation energy, is the minimum energy required for a chemical reaction to occur. learn the difference between threshold energy and activation energy in chemical reactions.learn the difference between threshold energy and activation energy in. Threshold Energy.
From www.youtube.com
Explain the terms Threshold energy 12 CHEMICAL Threshold Energyin chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they. This equation is valid if the energies are much less than rest mass energies of the involved particles.learn the definitions and differences of activation and threshold energies, and how they relate to chemical reactions and. Threshold energy. Threshold Energy.
From www.toppr.com
The energy profile for the reaction CO(g) + NO2(g) CO2(g) + NO(g) is Threshold Energy threshold energy, or activation energy, is the minimum energy required for a chemical reaction to occur.learn the difference between threshold energy and activation energy in chemical reactions. This equation is valid if the energies are much less than rest mass energies of the involved particles.in chemical reactions, the energy barrier corresponds to the amount of. Threshold Energy.
From www.researchgate.net
(a) Energy (Er in a.u.) and the threshold energy (E2s in a.u.) vs. 1 Z Threshold Energy Threshold energy is the energy of normal. learn the difference between threshold energy and activation energy in chemical reactions.learn how to calculate the threshold energy of a particle reaction using relativistic invariants and kinematics. threshold energy, or activation energy, is the minimum energy required for a chemical reaction to occur. This equation is valid if the. Threshold Energy.
From brainly.in
What is the relation between activation if energy and threshold energy Threshold Energylearn the difference between threshold energy and activation energy in chemical reactions. Threshold energy is the energy of normal.learn the definitions and differences of activation and threshold energies, and how they relate to chemical reactions and.in chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they.. Threshold Energy.
From www.researchgate.net
Displacement threshold energy and corresponding minimum electron Threshold Energylearn the definitions and differences of activation and threshold energies, and how they relate to chemical reactions and. learn the difference between threshold energy and activation energy in chemical reactions. threshold energy, or activation energy, is the minimum energy required for a chemical reaction to occur. Threshold energy is the energy of normal. This equation is valid. Threshold Energy.
From www.researchgate.net
Atomic structures and calculated displacement threshold energies for Threshold Energylearn the difference between threshold energy and activation energy in chemical reactions. This equation is valid if the energies are much less than rest mass energies of the involved particles. Threshold energy is the energy of normal.learn how to calculate the threshold energy of a particle reaction using relativistic invariants and kinematics.in chemical reactions, the. Threshold Energy.
From www.youtube.com
Threshold energy and activation energy YouTube Threshold Energylearn how to calculate the threshold energy of a particle reaction using relativistic invariants and kinematics.in chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they. threshold energy, or activation energy, is the minimum energy required for a chemical reaction to occur. Threshold energy is the energy. Threshold Energy.
From www.youtube.com
Video 8 Collision Theory and Threshold Energy YouTube Threshold Energylearn how to calculate the threshold energy of a particle reaction using relativistic invariants and kinematics. threshold energy, or activation energy, is the minimum energy required for a chemical reaction to occur. This equation is valid if the energies are much less than rest mass energies of the involved particles.learn the difference between threshold energy and. Threshold Energy.
From www.researchgate.net
Breakdown threshold energies calculated from different threshold Threshold Energylearn how to calculate the threshold energy of a particle reaction using relativistic invariants and kinematics. Threshold energy is the energy of normal.in chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they.learn the definitions and differences of activation and threshold energies, and how they relate. Threshold Energy.
From www.differencebetween.com
Difference Between Activation Energy and Threshold Energy Compare the Threshold Energy learn the difference between threshold energy and activation energy in chemical reactions.learn how to calculate the threshold energy of a particle reaction using relativistic invariants and kinematics. This equation is valid if the energies are much less than rest mass energies of the involved particles.in chemical reactions, the energy barrier corresponds to the amount of. Threshold Energy.
From www.periodic-table.org
What is Critical Energy Threshold Energy for Fission Definition Threshold Energylearn how to calculate the threshold energy of a particle reaction using relativistic invariants and kinematics. Threshold energy is the energy of normal. This equation is valid if the energies are much less than rest mass energies of the involved particles. learn the difference between threshold energy and activation energy in chemical reactions.learn the difference between. Threshold Energy.
From www.differencebetween.com
Difference Between Activation Energy and Threshold Energy Compare the Threshold Energy threshold energy, or activation energy, is the minimum energy required for a chemical reaction to occur.in chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they.learn how to calculate the threshold energy of a particle reaction using relativistic invariants and kinematics.learn the difference between. Threshold Energy.
From byjus.com
26. What is difference between threshold energy and activation energy? Threshold Energy learn the difference between threshold energy and activation energy in chemical reactions.learn the difference between threshold energy and activation energy in chemical reactions.learn how to calculate the threshold energy of a particle reaction using relativistic invariants and kinematics. threshold energy, or activation energy, is the minimum energy required for a chemical reaction to occur.. Threshold Energy.
From www.doubtnut.com
Consider the following figure for the reaction A+Brarr M+N Ans Threshold Energy learn the difference between threshold energy and activation energy in chemical reactions. Threshold energy is the energy of normal. threshold energy, or activation energy, is the minimum energy required for a chemical reaction to occur.learn the difference between threshold energy and activation energy in chemical reactions. This equation is valid if the energies are much less. Threshold Energy.